Concentrated Growth Factors vs. Leukocyte-and-Platelet-Rich Fibrin for Enhancing Postextraction Socket Healing. A Longitudinal Comparative Study
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Procedures
2.2. Outcome Variables
2.3. Statistical Analysis
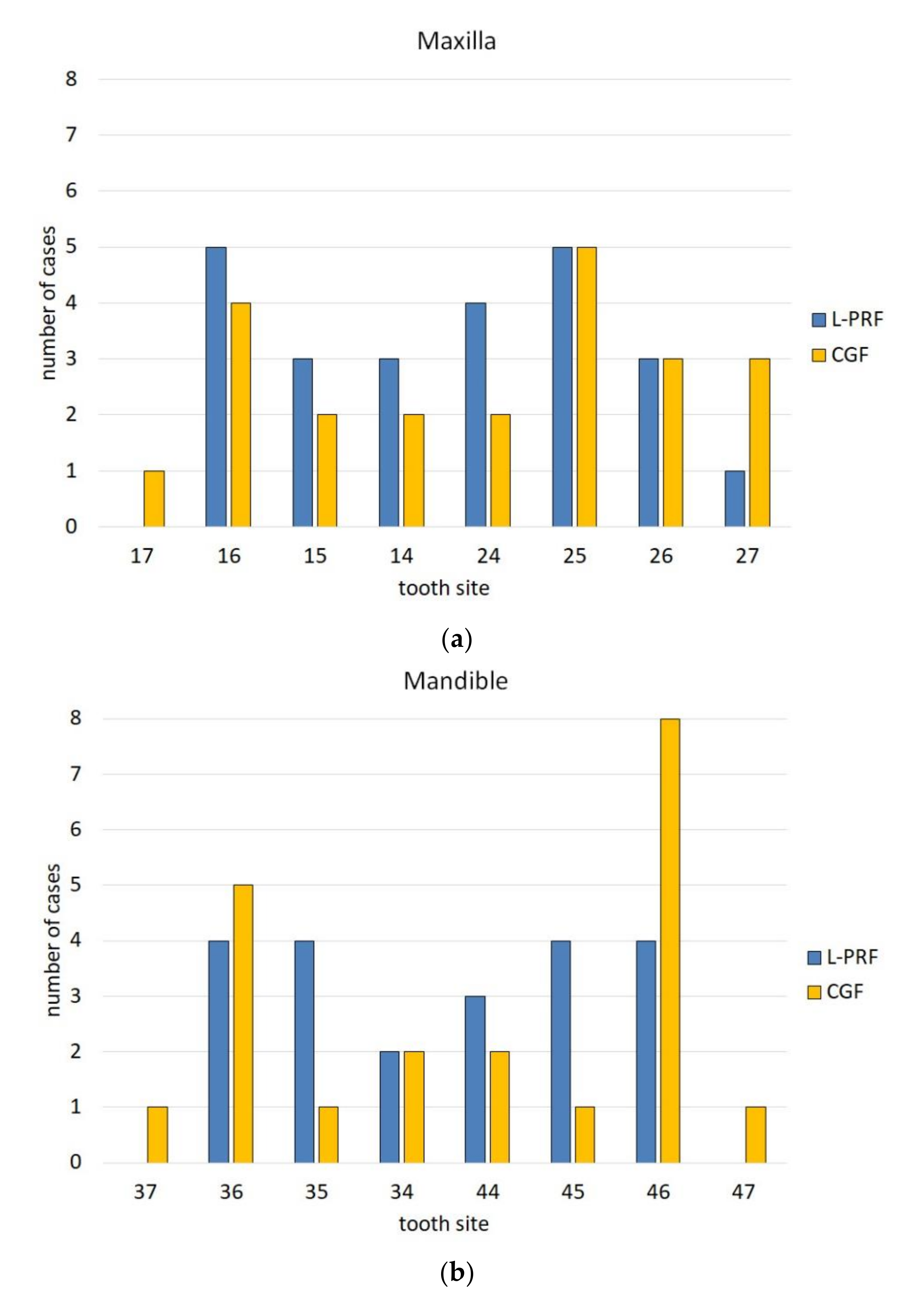
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anitua, E. The use of plasma-rich growth factors (PRGF) in oral surgery. Pract. Proced. Aesthet. Dent. 2001, 13, 487–493. [Google Scholar] [PubMed]
- Mihaylova, Z.; Mitev, V.; Stanimirov, P.; Isaeva, A.; Gateva, N.; Ishkitiev, N. Use of platelet concentrates in oral and maxillofacial surgery: An overview. Acta. Odontol. Scand. 2017, 75, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Feigin, K.; Shope, B. Use of platelet-rich plasma and platelet-rich fibrin in dentistry and oral surgery: Introduction and review of the literature. J. Vet. Dent. 2019, 36, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Mozzati, M.; Martinasso, G.; Pol, R.; Polastri, C.; Cristiano, A.; Muzio, G.; Canuto, R. The impact of plasma rich in growth factors on clinical and biological factors involved in healing processes after third molar extraction. J. Biomed. Mater Res. A 2010, 95, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Bortolin, M.; Taschieri, S. Is autologous platelet concentrate beneficial for post-extraction socket healing? A systematic review. Int. J. Oral Maxillofac. Surg. 2011, 40, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Ceresoli, V.; Lolato, A.; Taschieri, S. Effect of platelet concentrate on quality of life after periradicular surgery: A randomized clinical study. J. Endod. 2012, 38, 733–739. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Bucchi, C.; Lolato, A.; Corbella, S.; Testori, T.; Taschieri, S. Healing of postextraction sockets preserved with autologous platelet concentrates. A systematic review and meta-analysis. J. Oral Maxillofac. Surg. 2017, 75, 1601–1615. [Google Scholar] [CrossRef]
- Srinivas, B.; Das, P.; Rana, M.M.; Qureshi, A.Q.; Vaidya, K.C.; Ahmed Raziuddin, S.J. Wound healing and bone regeneration in postextractcon sockets with and without platelet-rich fibrin. Ann. Maxillofac. Surg. 2018, 8, 28–34. [Google Scholar] [CrossRef]
- Soto-Peñaloza, D.; Peñarrocha-Diago, M.; Cervera-Ballester, J.; Peñarrocha-Diago, M.; Tarazona-Alvarez, B.; Peñarrocha-Oltra, D. Pain and quality of life after endodontic surgery with or without advanced platelet-rich fibrin membrane application: A randomized clinical trial. Clin. Oral Investig. 2020, 24, 1727–1738. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-rich fibrin and soft tissue wound healing: A systematic review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Dragonas, P.; Katsaros, T.; Avila-Ortiz, G.; Chambrone, L.; Schiavo, J.H.; Palaiologou, A. Effects of leukocyte-platelet-rich fibrin (L-PRF) in different intraoral bone grafting procedures: A systematic review. Int. J. Oral Maxillofac. Surg. 2019, 48, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xu, Q.; Hou, J.; Wu, Y.; Liu, Y.; Li, R.; Pan, Y.; Zhang, D. Effect of platelet-rich fibrin on alveolar ridge preservation: A systematic review. J. Am. Dent. Assoc. 2019, 150, 766–778. [Google Scholar] [CrossRef]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scarì, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777. [Google Scholar] [CrossRef]
- Sohn, D.-S.; Heo, J.-U.; Kwak, D.-H.; Kim, D.-E.; Kim, J.-M.; Moon, J.-W.; Lee, J.-H.; Park, I.-S. Bone regeneration in the maxillary sinus using an autologous fibrin-rich block with concentrated growth factors alone. Implant Dent. 2011, 20, 389–395. [Google Scholar] [CrossRef]
- Durmuşlar, M.C.; Balli, U.; Dede, F.Ö.; Misir, A.F.; Bariş, E.; Kürkçü, M.; Kahraman, S.A. Histological evaluation of the effect of concentrated growth factor on bone healing. J. Craniofac. Surg. 2016, 27, 1494–1497. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, J.; Sun, Q.; Yan, S.; Wang, W.; Yang, P.; Song, A. One-year results evaluating the effects of concentrated growth factors on the healing of intrabony defects treated with or without bone substitute in chronic periodontitis. Med. Sci. Monit. 2019, 25, 4384–4389. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant Dent. 2016, 2, 19. [Google Scholar] [CrossRef]
- Isobe, K.; Watanebe, T.; Kawabata, H.; Kitamura, Y.; Okudera, T.; Okudera, H.; Uematsu, K.; Okuda, K.; Nakata, K.; Tanaka, T.; et al. Mechanical and degradation properties of advanced platelet-rich fibrin (A-PRF), concentrated growth factors (CGF), and platelet-poor plasma-derived fibrin (PPTF). Int. J. Implant Dent. 2017, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Gheno, E.; Palermo, A.; Rodella, L.F.; Buffoli, B. The effectiveness of the use of xenogeneic bone blocks mixed with autologous Concentrated Growth Factors (CGF) in bone regeneration techniques: A case series. J. Osseointegr. 2014, 6, 37–42. [Google Scholar]
- Mansour, P.; Kim, P. Use of concentrated growth factor (CGF) in implantology. Australas. Dent. Pract. 2010, 21, 162–176. [Google Scholar]
- Anitua, E.; Sánchez, M.; Nurden, A.T.; Nurden, P.; Orive, G.; Andía, I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006, 24, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, M.D.; Bortolin, M.; Taschieri, S.; Ceci, C.; Weinstein, R.L. Antimicrobial properties of platelet-rich preparations. A systematic review of the current pre-clinical evidence. Platelets 2016, 27, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Landry, R.G. Effectiveness of Benzydamine HC1 in the Treatment of Periodontal Post-Surgical Patients. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 1985. [Google Scholar]
- Del Fabbro, M.; Corbella, S.; Taschieri, S.; Francetti, L.; Weinstein, R. Autologous platelet concentrate for post-extraction socket healing: A systematic review. Eur. J. Oral Implantol. 2014, 7, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Marenzi, G.; Riccitiello, F.; Tia, M.; di Lauro, A.; Sammartino, G. Influence of leukocyte- and platelet-rich fibrin (L-PRF) in the healing of simple postextraction sockets: A split-mouth study. Biomed. Res. Int. 2015, 2015, 369273. [Google Scholar] [CrossRef]
- Singh, A.; Kohli, M.; Gupta, N. Platelet rich fibrin: A novel approach for osseous regeneration. J. Maxillofac. Oral Surg. 2012, 11, 430–434. [Google Scholar] [CrossRef]
- de Almeida Barros Mourão, C.F.; de Mello-Machado, R.C.; Javid, K.; Moraschini, V. The use of leukocyte- and platelet-rich fibrin in the management of soft tissue healing and pain in post-extraction sockets: A randomized clinical trial. J. Cranio Maxillofac. Surg. 2020, 48, 452–457. [Google Scholar] [CrossRef]
- Miron, R.J.; Xu, H.; Chai, J.; Wang, J.; Zheng, S.; Feng, M.; Zhang, X.; Wei, Y.; Chen, Y.; de Almeida Barros Mourão, C.F.; et al. Comparison of platelet-rich fibrin (PRF) produced using 3 commercially available centrifuges at both high (~700 g) and low (~200 g) relative centrifugation forces. Clin. Oral Investig. 2020, 24, 1171–1182. [Google Scholar] [CrossRef]
- Asmael, H.M.; Jamil, F.A.; Hasan, A.M. Novel application of platelet-rich fibrin as a wound healing enhancement in extraction sockets of patients who smoke. J. Craniofac. Surg. 2018, 29, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Ustaoğlu, G.; Göller Bulut, D.; Gümüş, K.Ç. Evaluation of different platelet-rich concentrates effects on early soft tissue healing and socket preservation after tooth extraction. J. Stomatol. Oral Maxillofac. Surg. 2019, in press. [Google Scholar] [CrossRef]
- Özveri Koyuncu, B.; Işık, G.; Özden Yüce, M.; Günbay, S.; Günbay, T. Effect of concentrated growth factor (CGF) on short-term clinical outcomes after partially impacted mandibular third molar surgery: A split-mouth randomized clinical study. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Salman, B.; Abdul Razak, N.H.; Qabbani, A.A.; Samsudin, A.R. The Efficacy of concentrated growth factor in the healing of alveolar osteitis: A clinical study. Int. J. Dent. 2020, 2020, 9038629. [Google Scholar] [CrossRef]
- Kamal, A.; Salman, B.; Razak, N.H.A.; Samsudin, A.B.R. A Comparative clinical study between concentrated growth factor and low-level laser therapy in the management of dry socket. Eur. J. Dent. 2020, 14, 613–620. [Google Scholar] [CrossRef]
- Schär, M.O.; Diaz-Romero, J.; Kohl, S.; Zumstein, M.A.; Nesic, D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin. Orthop. Relat. Res. 2015, 473, 1635–1643. [Google Scholar]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Jimbo, R.; Barbe, G.; Del Corso, M.; Inchingolo, F.; Sammartino, G. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte-and platelet-rich fibrin (L-PRF). Curr. Pharm. Biotechnol. 2012, 13, 1145–1152. [Google Scholar] [CrossRef]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jiménez, P.; Corso, M.D.; Kang, B.-S.; Nally, M.; Lanata, N.; Wang, H.-L.; Quirynen, M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte-and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef]
- Lee, H.-M.; Shen, E.-C.; Shen, J.T.; Fu, E.; Chiu, H.-C.; Hsia, Y.-J. Tensile strength, growth factor content and proliferation activities for two platelet concentrates of platelet-rich fibrin and concentrated growth factor. J. Dent. Sci. 2020, 15, 141–146. [Google Scholar] [CrossRef]
- Krishnakumar, D.; Mahendra, J.; Ari, G.; Perumalsamy, R. A clinical and histological evaluation of platelet-rich fibrin and CGF for root coverage procedure using coronally advanced flap: A split-mouth design. Indian J. Dent. Res. 2019, 30, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Yu, Y.; Han, J.; Shi, D.; Sun, W.; Zhang, D.; Chen, L. Quantification of growth factors in advanced platelet-rich fibrin and concentrated growth factors and their clinical efficacy as adjunctive to the GTR procedure in periodontal intrabony defects. J. Periodontol. 2020, 91, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Torul, D.; Omezli, M.M.; Kahveci, K. Evaluation of the effects of concentrated growth factors or advanced platelet rich-fibrin on postoperative pain, edema, and trismus following lower third molar removal: A randomized controlled clinical trial. J. Stomatol. Oral Maxillofac. Surg. 2020, in press. [Google Scholar] [CrossRef] [PubMed]


| CGF | PRF | P-Value | |
|---|---|---|---|
| Maxilla/mandible (n. teeth) | 24/21 | 24/21 | P = 1 |
| Reason for extraction (n.teeth) - advanced caries | 28 | 26 | P = 0.89 |
| - Periodontal disease | 15 | 16 | |
| - Tooth fracture | 2 | 3 | |
| Baseline alveolar size VP/L, mean ± SD (mm) | 9.42 ± 2.71 | 9.22 ± 1.81 | P = 0.68 |
| Baseline alveolar size MD, mean ± SD (mm) | 9.51 ± 4.03 | 9.18 ± 2.88 | P = 0.65 |
| Intra-operative complications (number) | 3 | 4 | P = 0.69 |
| Post-operative complications (number) | 0 | 0 | N.A. |
| Healing index at day 7 (score) | 5.22 ± 1.36 | 5.40 ± 1.29 | P = 0.53 |
| Vestibulo-Palatal/Lingual Change, mm (% Closure Respect to Baseline) | Mesio-Distal Change, mm (% Closure Respect to Baseline) | |||||
|---|---|---|---|---|---|---|
| Group | 0–7 Days | 0–14 Days | 0–21 Days | 0–7 Days | 0–14 Days | 0–21 Days |
| CGF | 1.89 ± 1.79 (20.0%) | 4.24 ± 1.90 (45.0%) | 7.62 ± 2.23 (80.9%) | 2.44 ± 1.90 (25.7%) | 4.98 ± 2.50 (52.3%) | 7.73 ± 3.23 (81.3%) |
| L-PRF | 1.09 ± 1.82 (11.8%) | 3.62 ± 1.95 (39.3%) | 7.29 ± 2.02 (79.0%) | 1.76 ± 2.39 (19.1%) | 4.38 ± 2.60 (47.7%) | 7.62 ± 3.04 (83.1%) |
| P-value | 0.041* | 0.108 | 0.349 | 0.149 | 0.210 | 0.820 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozzati, M.; Gallesio, G.; Tumedei, M.; Del Fabbro, M. Concentrated Growth Factors vs. Leukocyte-and-Platelet-Rich Fibrin for Enhancing Postextraction Socket Healing. A Longitudinal Comparative Study. Appl. Sci. 2020, 10, 8256. https://doi.org/10.3390/app10228256
Mozzati M, Gallesio G, Tumedei M, Del Fabbro M. Concentrated Growth Factors vs. Leukocyte-and-Platelet-Rich Fibrin for Enhancing Postextraction Socket Healing. A Longitudinal Comparative Study. Applied Sciences. 2020; 10(22):8256. https://doi.org/10.3390/app10228256
Chicago/Turabian StyleMozzati, Marco, Giorgia Gallesio, Margherita Tumedei, and Massimo Del Fabbro. 2020. "Concentrated Growth Factors vs. Leukocyte-and-Platelet-Rich Fibrin for Enhancing Postextraction Socket Healing. A Longitudinal Comparative Study" Applied Sciences 10, no. 22: 8256. https://doi.org/10.3390/app10228256
APA StyleMozzati, M., Gallesio, G., Tumedei, M., & Del Fabbro, M. (2020). Concentrated Growth Factors vs. Leukocyte-and-Platelet-Rich Fibrin for Enhancing Postextraction Socket Healing. A Longitudinal Comparative Study. Applied Sciences, 10(22), 8256. https://doi.org/10.3390/app10228256







